April 3, 2020 feature
Isolating an elusive phosphatetrahedrane

Thamarasee Jeewandara
contributing writer
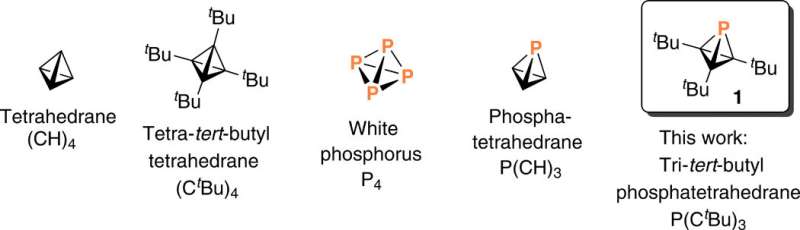
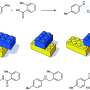
A research team in the Department of Chemistry at the Massachusetts Institute of Technology (MIT), Cambridge U.S., explored a synthetic pathway to generate a framework. During the synthetic route, the team replaced a single carbon vertex with another p-block element within a highly strained molecule. Highly strained molecules possess unusually acute bond angles at carbon and are species of high energy. In order to replace a single carbon vertex for less strain in this work, Martin-Louis Y. Riu and colleagues selected phosphorous due to its stable, tetrahedral molecular form. They accomplished the task through of [H(F)P(CtBu)3] generated during the synthetic route. The team isolated a 19 percent yield of the Tri-tert-butyl phosphatetrahedrane [P(CtBu)3] product of interest as a low-melting, volatile and colorless solid. They characterized the product spectroscopically and with single-crystal X-ray diffraction to confirm the tetrahedral nature of the molecule's PC3 core and noted the unexpected thermal stability of the molecule.
Strained cages such as tetrahedrane are interesting structural components used to design materials. Although the parent tetrahedrane molecule has remained elusive, it is a and chemists aim to successfully isolate molecules containing the tetrahedrane core with four carbon atoms and encage it to synthesize new materials. In a complementary approach, researchers can into the tetrahedral core including phosphorous, known as "the carbon copy" due to its approximation to carbon—based on electronegativity and the ability to form multiple bonds—which forms the basis of . In where molecules contain unusually acute bond angles at carbon, Riu et al. replaced a carbon atom of tetrahedrane with phosphorous to yield a stable molecular entity. The potential to create a phosphatetrahedrane is logical due to the tetrahedral nature of the P4 molecule—the only of elemental phosphorous. For instance, theoretical work has predicted that phosphatetrahedrane will after gas-phase protonation (addition of a proton or hydrogen).
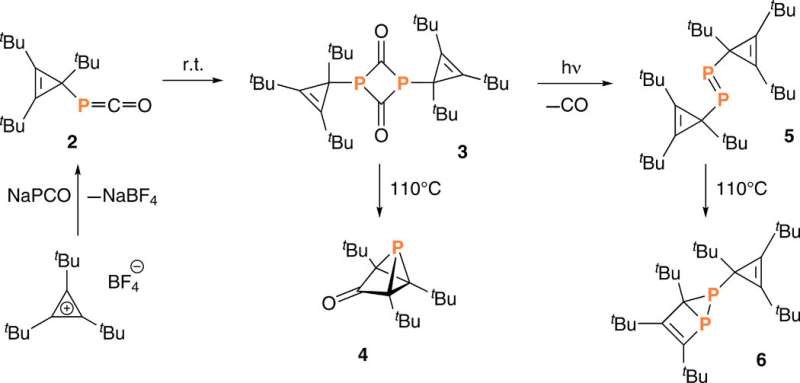
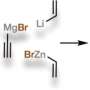
Since substituting bulky groups is key to tetrahedranes, in this work Riu et al. selected P(CtBu)3 as their target molecule. Based on their experience with , the team prepared a compound, AP(CtBu)3 where A was anthracene or C14H10 and analogous to . During the process, the team deprotonated a and collected the product by filtration after precipitating the crude reaction mixture, to eventually produce as a ninth product in the synthetic route. They characterized compound nine with single-crystal X-ray diffraction to reveal its molecular structure. The cyclopropenyl phosphine product was thermally stable at least to its melting point of 1300C and Riu et al. conducted photochemical experiments to induce anthracene elimination.
After brief periods of irradiation, they produced a species containing a signal, which they identified as one component of the desired phosphatetrahedrane molecule (named as compound 1) within a complex mixture. Extended periods of irradiation led to a loss of the intriguing high-field NMR signal that represented the product, while showing increased complexity of the reaction mixture. Since halides (chloride, bromide, fluoride) can also induce elimination of anthracene, the team studied an alternative strategy to generate HXP(CtBu)3 cyclopropenyl halophosphines from compound nine, as to form the of interest. The team treated compound nine to eliminate the anthracene molecule and formed HXP(CtBu)3, where X could either be fluoride to form compound 10, or chloride to form compound 11, which they identified using NMR.
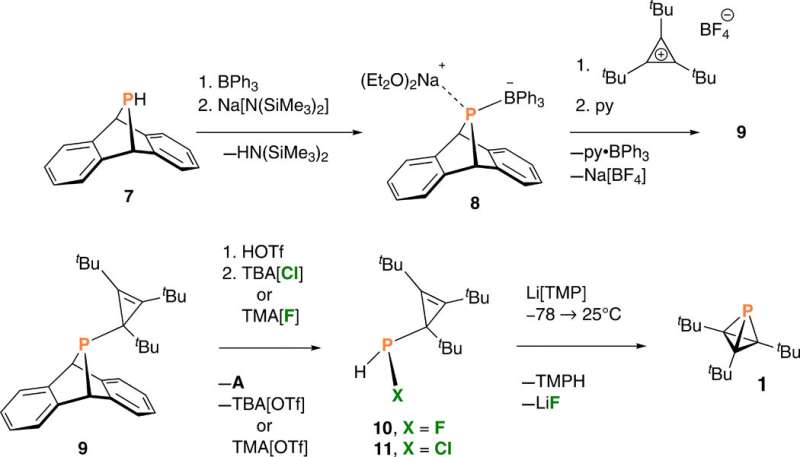
Dehydrohalogenation of compound 10 –fluorophosphine was efficient and reproducible to produce phosphatetrahedrane (compound 1) – the structure of interest. The team optimized the protocol to deliver compound 1 as the major product and characterized the result with NMR signals to confirm its purity/identity. Riu et al. first obtained crude samples of the phosphatetrahedrane product as a pale yellow oil, which they purified through a silica plug to deliver colorless solid samples. They then credited the low yield of the isolated compound to volatility during isolation and purification.
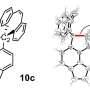
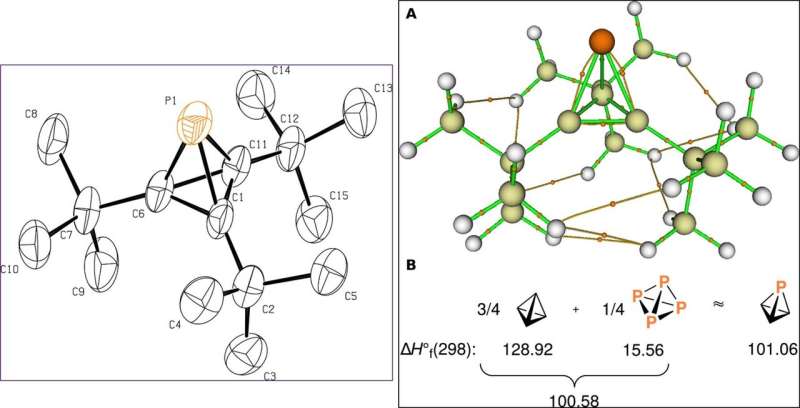
![Molecular structures of key intermediates obtained from single-crystal x-ray diffraction experiments. (A) Drawing of Na[8] with thermal ellipsoids shown at the 50% probability level. Hydrogen atoms have been omitted. (B) Drawing of compound 9 with thermal ellipsoids shown at the 50% probability level. Hydrogen atoms have been omitted. Science Advances, doi: 10.1126/sciadv.aaz3168 Isolating an elusive phosphatetrahedrane](https://scx1.b-cdn.net/csz/news/800a/2020/3-isolatingane.jpg)
The scientists ultimately grew crystals of the product of interest—phosphatetrahedrane for X-ray diffraction investigations and used to overcome volatility for smoother product development. Using the data, they determined the structure of phosphatetrahedrane, which agreed well with those predicted using quantum chemical calculations. The final compound showed considerable thermal stability and stability in air (at room temperature) for half hour, although they were unstable under 254 nm ultraviolet irradiation. Riu et al. used quantum chemical calculations to illuminate the bonding and explained the stability of the molecular framework, while illustrating how three bulky substitutions were sufficient to produce .
In this way, Martin-Louis Y. Riu and colleagues synthesized tri-tert-butyl phosphatetrahedrane to produce proof of existence of a molecule with the smallest sum of bond angles conceivable for a trivalent phosphorous atom. To successfully synthesize phosphatetrahedrane, the team relied on novel phosphinidenoid reaction chemistry that remains to be explained in mechanistic detail. The strategy described in this work will be applicable to other strained synthetic targets.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Martin-Louis Y. Riu et al. Isolation of an elusive phosphatetrahedrane, Science Advances (2020).
David J. Liptrot et al. London dispersion forces in sterically crowded inorganic and organometallic molecules, Nature Reviews Chemistry (2017).
Gabriele Hierlmeier et al. Di‐ tert ‐butyldiphosphatetrahedrane: Catalytic Synthesis of the Elusive Phosphaalkyne Dimer, Angewandte Chemie International Edition (2019).
Journal information: Science Advances , Angewandte Chemie International Edition
© 2020 Science X Network